Axial overlapping of two atoms forms a sigma bond while lateral overlapping of two atomic orbitals forms a sigma bond. Describe a sigma bond.

Sigma And Pi Bonds Definition And Detailed Explanation
Covalent bonds can be single double and triple bonds.
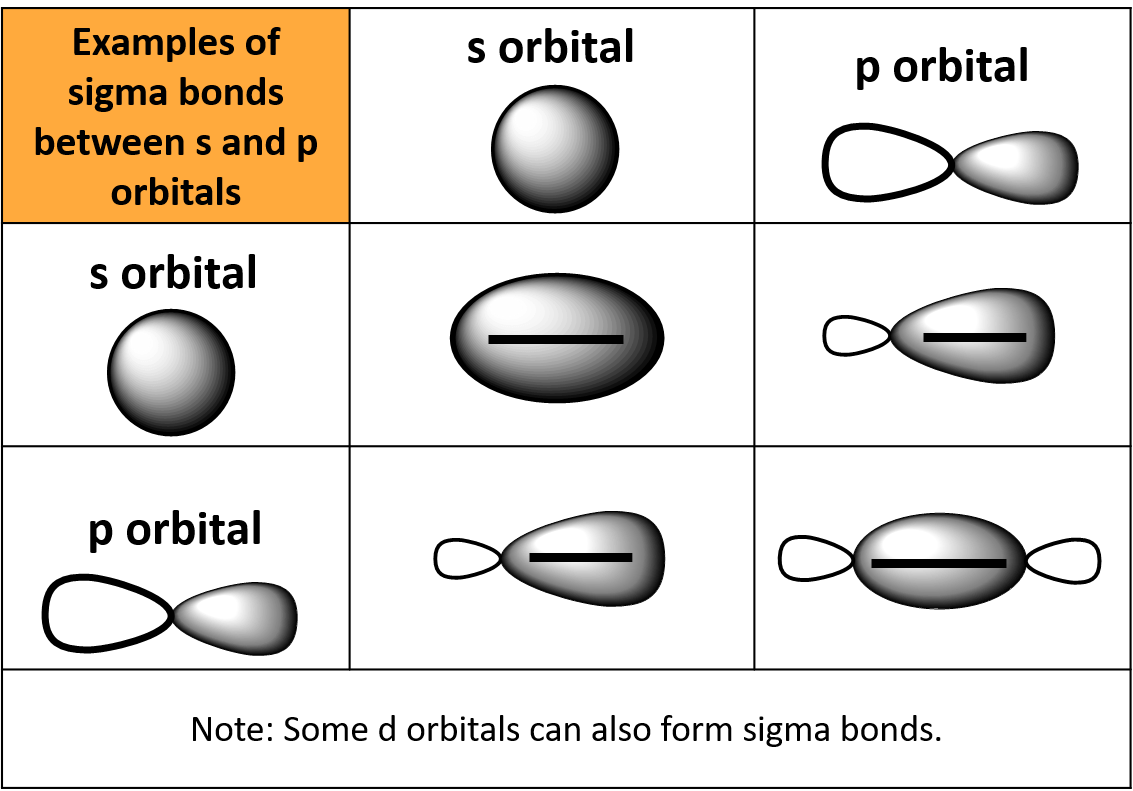
. Side-by-side overlap of p orbitals make. This type of bond has ____ regions of electron density. Single bonds occur when two electrons are shared and are composed of one sigma bond between the two atoms.
Sigma bonding can be a bonding interaction or an antibonding interaction. A covalent bond formed by collinear or coaxial ie. This bond has its highest electron density.
Anything that is bonded by a single covalent bond. Thus covalent bonds are classified into sigma bond and pi bond based on the type of overlapping. Bonding interaction results by the overlapping of two atomic orbitals in the same phase whereas antibonding interaction occurs by the.
A sigma bond is a covalent bond which is formed by the head on overlap of two atomic orbitals. In organic molecules a single C-C bond is a sigma bond while a multiple C-C bond is composed of one sigma bond together with pi or other bonds that is a double bond has one sigma plus one pi bond and a triple bond has one sigma plus two pi bonds. In a sigma bond the electron pair occupies an orbital a region of space associated with a particular value of the energy of the systemlocated mainly between the two atoms and.
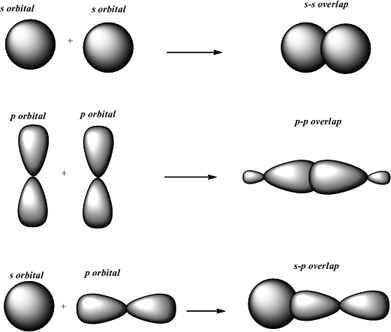
Thus s-s overlap always forms a sigma bond. In a line of internuclear axis overlapping of an atomic orbital is known as a sigma bond. By this definition common forms of.
This is the key difference between sigma and pi bond. See the answer See the answer done loading. C s orbital overlapping with the side of a p orbital.
Sigma bonding is most simply defined for diatomic molecules using the language and tools of symmetry groups. E the attraction between 2 metal atoms. Which of the following statements correctly describe a sigma bond.
They are formed by head-on overlapping between atomic orbitals. This type of covalent bond is formed by the overlap of bonding orbitals. The covalent bond formed by the axial overlap of atomic orbitals is called a sigma bond.
In chemistry sigma bonds are the strongest type of covalent chemical bond. Difference Between Sigma and Pi Bond. CC bonds and CH bonds of propene.
A pi bond is a type of covalent bond that exists between atoms where the electrons are on top and bottom of the axis connecting the nuclei of the joined atoms. E None of the above contain both ionic and covalent bonds. The symbol σ is used to adequately describe the bond that exists in a molecule.
Add your answer and earn points. A pi bond bond is a bond formed by the overlap of orbitals in a side-by-side fashion with the electron density concentrated above and below the plane of the nuclei of the bonding atoms. - single bonds are sigma bonds.
A sigma bond and pi bond. Which are stronger bonds. Formation of Sigma Bond.
View the full answer. Sigma bonds are formed by head-on overlapping between atomic orbitals. A sigma bond bond is a bond formed by the overlap of orbitals in an end-to-end fashion with the electron density concentrated between the nuclei of the bonding atoms.
The combination of overlapping orbitals can be s-s s-p z or p z -p z. Sigma bond σ bond. Describe a sigma bondA overlap of two f orbitalsB end to end overlap of p orbitalsC s orbital overlapping with the side of a p orbitalD side by side overlap of p orbitalsE p orbital overlapping with a d orbital 1 See answer kolbiking3883 is waiting for your help.
While sigma and pi bonds are used to describe some features of covalent. What can only form sigma bonds. 9 Describe a sigma bond.
A bond formed by the overlap of two s orbitals or the end-to-end overlap of two orbitals that have p character is called a _____ bond. B end to end overlap of p orbitals. Some examples are.
B the transfer of electrons from one atom to another. An ionic bond is best described as. In this formal approach a σ-bond is symmetrical with respect to rotation about the bond axis.
It is formed by the parallel or lateral overlapping of the atomic orbitals. This type of covalent bond is formed by head-on positive same phase overlap of atomic orbitals along the internuclear axis. Sigma bond in chemistry a mechanism by which two atoms are held together as the result of the forces operating between them and a pair of electrons regarded as shared by them.
A covalent bond formed by overlap of atomic orbitals andor hybrid orbitals along the bond axis ie along a line connected the two bonded atoms. A overlap of two f orbitals. S orbitals are non-directional hence they can overlap in any side.
The electronsparticipating in a σ bond are commonly referred to as σ electrons. Sigma bonds are the strongest covalent bonds owing to the direct overlapping of the participating orbitals. They are formed based on the orbitals of the bonding electrons between two atoms ie a sigma pond forms between two electrons in the s orbital.
D side by side overlap of p orbitals. E p orbital overlapping with a d orbital. Pi bonds are a weaker type of covalent interactions and result from the overlap of two lobes of the interacting atomic orbitals above and below the orbital axis.
Single bond is made up of. A the sharing of electrons. O end-to-end overlap of atomic orbitals only side-to-side overlap of hybrid orbitals only side-to-side overlap of atomic orbitals only O end-to-end overlap of atomic orbitals andor hybrid orbitals.
What are required for double and triple bonds. In order to form sigma bond p orbitals must lie along the internuclear axis. A sigma bond is a single bond a double bond is a sigma and pi bond and a triple bond is a sigma bond and two pi bonds.
A sigma bond is a covalent connection produced by collinear or coaxial overlapping of an atomic orbital in a line of the internuclear axis. Sigma and pi are two type of bonds formed due to the overlapping two atomic orbitals. For example the methane molecule contains 4 C-H sigma bonds.
The electrons that participate in a σ bond are referred to as σ electrons. D the attraction between 2 nonmetal atoms. If two four or six electrons are shared the number of bonds formed will be one two or three respectively.
100 1 rating Sigma bond is formed by the end to end overlap of atomic. Due to the direct overlapping of the involved orbitals sigma bonds are the strongest covalent bonds. C the attraction that holds the atoms together in a polyatomic ion.
This type of covalent bond is formed by the end to end head-on overlap of bonding orbitals along the internuclear axis.
Definition Of Sigma And Pi Bonds Chegg Com

Sigma And Pi Bonds Brilliant Math Science Wiki

0 Comments